uganda
research consortia
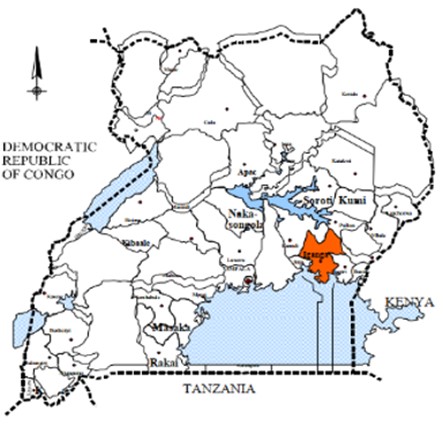
Iganga-Mayuge Health and Demographic Surveillance Site (IMHDSS)
IMHDSS serves as a population-based research platform where demographic and health data are collected from a defined population over time. IMHDSS will contribute valuable data for TB research and surveillance efforts. The site covers a contiguous and clearly demarcated area of 155km2, a part of Iganga and a part of Mayuge districts. It is made up of 65 villages in seven sub-counties and it is enclosed by 16 health centres and a hospital. Currently, the HDSS covers a population of 90,000 people from 17,000 households, about 59% living in rural areas.
Jinja Regional Referral Hospital
JRRH is a regional referral hospital for Eastern Uganda, it offers outpatient, inpatients and specialized healthcare services including TB case management. RePORT activities at this site will involve clinical research, patient care, and capacity building. The site is actively enrolling study participants in a number of diagnostic studies including Novel and Optimized Diagnostics for Pediatric TB in Endemic Countries and Accuracy of Novel Diagnostic Tests for Detection of Tuberculosis in Adults (FEND-TB).
Integrated Biorepository of H3Africa Uganda (IBRH3AU)
IBRH3AU is an integrated biorepository at Makerere University College of Health Sciences under the NIH’s H3Africa initiative. IBRH3AU provides a resource of well-characterized and annotated high quality biospecimens for future use by researchers as well as trainers. This resource serves both communicable and non-communicable disease researchers in Africa. The ultimate goal of IBRH3AU is to improve the prevention, diagnosis, and treatment of illness and the promotion of health throughout society. All activities are done in adherence to current SOPs and GCLP standards. The quality assurance program covers the pre-analytical, analytical, and post-analytical phases. This is through standard procedures like sample rejection/acceptance, routinely monitored Turn-Around-Time, Internal Quality Controls (IQCs), External quality assessment (EQA) proficiency programs, Blind/Split sample testing, etc. IBRH3AU is currently in its advanced stage preparations for SANAS/SADCAS accreditation. The biorepository is also equipped with state-of-the-art Genomics, Molecular and Immunology laboratories that process biospecimens and add value. IBRH3AU will be key in the long-term storage and shipment of biospecimens collected under the REPORT protocol. IBRH3AU currently supports research studies in East and Central Africa.
The Genomics, Molecular, and Immunology Laboratories (GMI Labs)
GMI labs are diagnostic, research, and training facilities, focused on both infectious and non-infectious diseases or conditions. The GMI Laboratories provide laboratory services to all healthcare providers, including physicians, researchers, epidemiologists, students, and all healthcare policymakers, for the benefit of patients and the global community. GMI offers quality diagnostic, training, and research services using the most advanced Genomics, Molecular, and Immunology techniques while complying with the ISO 15189:2012 requirements for medical laboratories.
CAP-accredited Mycobacteriology Laboratory (BSL-3)
The BSL-3 lab is a College of American Pathologists (CAP) – accredited Biosafety Level (BSL)-3 culture facility. It is a site for multinational tuberculosis research initiatives namely: the Division of AIDS Clinical Trials regional TB diagnostic laboratory (DAIDS/ACTG/RTBDL) and the International Maternal, Paediatric, Adolescent AIDS Clinical Trials (IMPAACT) Network, and the Global Alliance for TB drug development studies. This facility has vast experience in clinical trials including those for novel tuberculosis drugs for treating MDR-TB for example, the laboratory participated in the BPaMZ regimen for MDR-TB treatment. Other clinical trials include those for both susceptible and resistant TB with study numbers in public databases [NCT02410772 (TBTC study 31, S31/A5349), NCT02193776 (NC-005-(J-M-Pa-Z), NCT01380080 (REMEMBER, ACTG 5274), IMPAACT P1078 (DAIDS ID 10732), ISRCTN63579542, NCT02342886 (NC-006-(M-Pa-Z)) among others. It has participated in several diagnostic evaluation studies some of which have made it to WHO policies most recently being the multi- country Xpert ULTRA evaluation study. The CAP accredited BSL-3 offers clinical and research TB diagnostic services according to WHO and international guidelines.
Uganda Supra National Reference Laboratory (SRL)
Bacteriological Investigation Unit in the late 1950’s under the then East African Community. The laboratory participated in anti TB clinical trials and drug toxicities under the then British Medical Research Council (MRC). After the collapse of the East African Community in 1970’s, the laboratory reverted to the line ministries. Its name changed to Central Tuberculosis laboratory (CTBL) in 1980’s and National Tuberculosis Reference Laboratory in the 1990’S. The Uganda National Tuberculosis Reference Laboratory established under the National Tuberculosis and Leprosy Programme (NLTP) of the Ministry of Health (MoH) received accreditation from the WHO in April 2013, making it the first SRL in East Africa, and the second in Sub-Saharan Africa to achieve this status. SRL supports the integration of quality TB diagnostic services for the purpose of providing prompt and accurate results to patients according to the International Standard of Care with national laboratory strategic plans, incorporating cross cutting laboratory issues including supply management, specimen transport, and referral and human resource development. SRL advocates for TB laboratory worker protection with use of current WHO TB bio-safety recommendations. It supports the development of monitoring and evaluation indicators starting with a good data management system. SRL is part of the National TB and Leprosy Program Uganda (NTLP).
IDI-African Center of Excellence in Bioinformatics & Data-intensive Sciences (ACE)
The Infectious Diseases Institute (IDI), Makerere University’s College of Computing & Health Sciences (CHS) in partnership with the US Government National Institute of Allergy and Infectious Diseases (NIAID) and the Office of Cyber Infrastructure and Computational Biology (NIH/NIAID/OCICB) established the African Centre of Excellence in Bioinformatics & Data Sciences, one of the 2 such centres on the African continent. ACE is focused on bioinformatics and data-intensive research in the context of infectious diseases, including TB. It provides expertise in data analysis, informatics, and bioinformatics to support TB research and improve data management.