Highlighting RePORT Indonesia
RePORT Indonesia has been an important collaborator with RePORT International protocols. As we await decisions regarding prospective recruitment, I appreciate their investigators’ contributions to the Annual Meeting.
Background
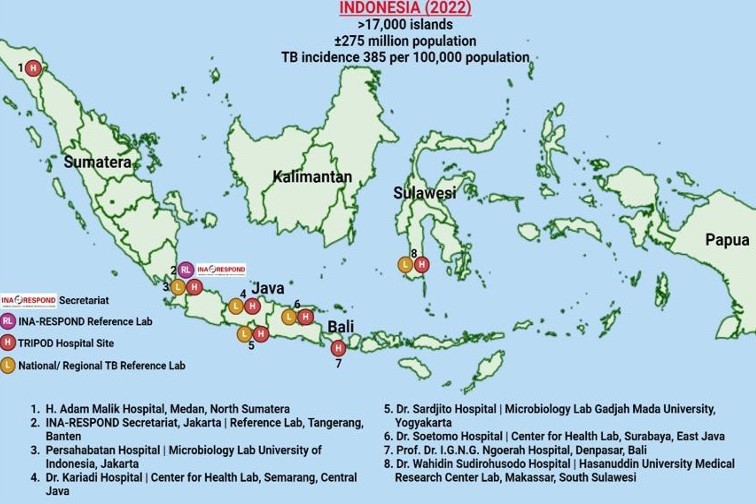
RePORT Indonesia is an activity of the Indonesia Research Partnership on Infectious Diseases (INA-RESPOND), a government-to-government clinical research partnership between the U.S. National Institute of Allergy and Infectious Diseases (NIAID) and the Health Services Directorate (Direktorat Jenderal Pelayanan Kesehatan/Yankes) of the Indonesia Ministry of Health. The INA-RESPOND Secretariat, based in Jakarta, has coordinated infectious disease studies at 19 large referral hospitals across Indonesia, including 7 tuberculosis (TB) referral centers. Research priorities of the INA-RESPOND partnership align with those of NIAID and the Ministry of Health, which continues to identify TB as a national priority disease. Research activities are supported by dedicated teams of scientific, clinical, operations, and administrative staff in Indonesia and technical staff from the NIAID Division of Clinical Research in the U.S.
RePORT Indonesia, led by Principal Investigator Prof. Dr. Erlina Burhan, Sp.P (K), MSc, PhD, completed a prospective observational cohort study on TB from 2017-2021 called TB Research of INA-RESPOND on Drug Resistance (TRIPOD). That study incorporated elements from the RePORT International Common Protocol Cohort A for collecting data and biospecimens using standardized methods and agreed upon time points. The first site activation was in January 2017, and enrollment of 490 adult presumptive TB patients completed in November 2018, with follow-up of the last participant concluding in April 2021. Patients were assigned to DS-TB or MDR- TB treatment regimens and followed for treatment outcomes every month for six months, followed by every two months until the end of treatment. The study involved 7 TB referral hospitals on the islands of Sumatera, Java, Bali, and Sulawesi, as well as 5 additional TB laboratories for microbiological testing.
Accomplishments
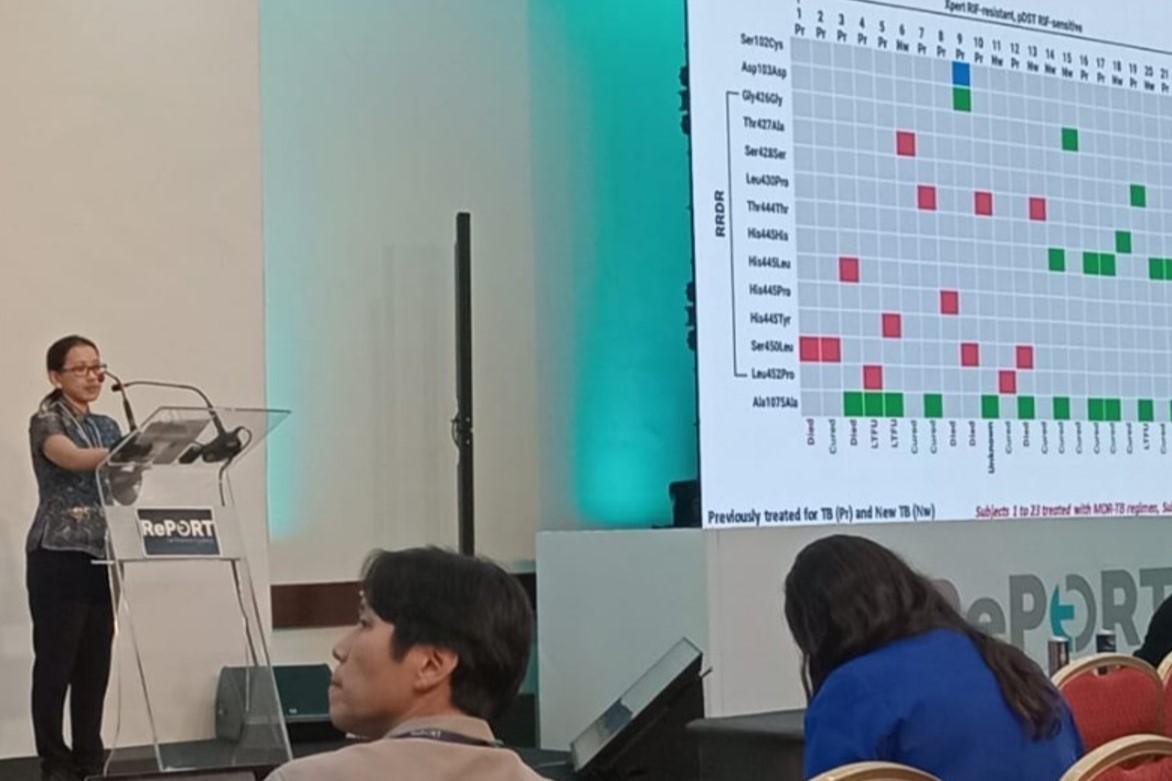
All study sites used the same protocol and case report form. The TRIPOD data were managed by the Data Manager team using electronic data capture and clinical data management systems. Based on TRIPOD data, RePORT Indonesia successfully published two main articles, underscoring the need for TB research in Indonesia. The first publication reported a significantly higher proportion of MDR-TB among both newly diagnosed and previously treated TB cases than what was noted in the WHO Global TB Report, emphasizing the ongoing threat of MDR-TB transmission and the need for continuous TB surveillance in Indonesia. The second publication assessed the performance of TB diagnostic tests, supporting the National TB policy in expanding the use of Xpert MTB/RIF for diagnosis. The discordant results in Rifampicin resistance between Xpert MTB/RIF and phenotypic DST led the team to explore MTB genomic sequencing, highlighting the importance of epidemiological data on anti-TB drug-gene mutations. Currently, the manuscript on TRIPOD treatment outcomes is under review in the IJTLD Open.
Approximately 8,600 specimens (including serum, plasma, whole blood, whole blood in PAXgene RNA tubes, PBMC, urine, saliva, sputum, and Mycobacterium tuberculosis (MTB) isolates) from all participants and visits were stored at the INA-RESPOND Reference Laboratory. Most specimens were kept in a freezer at -80°C, with PBMC specimens stored in a liquid nitrogen dewar at -150°C. RePORT International supports the laboratory team in implementing FreezerPro for efficient specimen management. RePORT Indonesia encouraged researchers to collaborate and utilize these repository specimens. One such collaboration, with fungal experts Retno Wahyuningsih and David W. Denning, led to the publication titled “The Seroprevalence of Anti-Histoplasma capsulatum IgG Among Pulmonary TB Patients,” using repository sera.
RePORT Indonesia aims to provide evidence-based recommendations for the national tuberculosis programs. Additionally, data and specimens collected from this study will be useful for planning future TB research studies, such as evaluating novel diagnostic tests, evaluating biomarkers of response to treatment, and identifying new therapies. RePORT Indonesia actively participates in the RePORT International Protocols for Epidemiology of TB Progression and Outcomes and Treatment Response Biomarkers. The team completed data harmonization and, in November, successfully shipped specimens for the TB biomarker study to Rutgers, navigating the complex material transfer agreement (MTA) process. These experiences are invaluable for future collaborations within the RePORT Consortium.
Challenges
RePORT Indonesia is fortunate to have a network of 19 hospitals for research collaboration, a professional Secretariat as the coordinating center, and skilled lab technologists in the INA-RESPOND Reference Laboratory. However, challenges remain, including a limited number of investigators with TB expertise and the early stage of genomic, metabolomic, and proteomic TB research development in Indonesia. Additionally, INA-RESPOND is undergoing a significant reorganization under the Ministry of Health (MoH), with clinical research being restructured into the Clinical Research Center (CRC) and Clinical Research Units (CRUs) across 17 MoH hospitals. INA-RESPOND will operate as part of the Sulianti Saroso Infectious Disease Hospital CRU. With this transition expected to be completed by 2025, there is currently no active funding for TB studies, prompting the team to seek external funding and collaboration opportunities. Building in-country capacity and maintaining active participation in the RePORT Consortium are therefore essential for advancing TB research in Indonesia and contributing to global efforts to end TB.
What's new
Celebrating Dr. Rajita R. Bhavaraju
Celebrating Dr. Rajita R. BhavarajuLifetime Service Award RecipientCelebrating Dr. Rajita R. Bhavaraju: Lifetime Service Award RecipientI am pleased to announce that "our own" Rajita Bhavaraju, PhD, MPH, Director of Global Operations for the TB-RePORT International...
Update on Biomarkers Study
update on biomarkers studyAnalysis of Host Biomarkers Associatedwith Adverse TB Treatment Outcomes Across RePORT International SitesAnalysis of Host Biomarkers Associatedwith Adverse TB Treatment Outcomes Across RePORT International Sites Sheetal Verma
RiCC Leadership Group
RICC International leadershipCopenhagen | November 18, 2025Meeting Highlights Copenhagen | November 18, 2025 The RePORT International Leadership Group met in Copenhagen on November 18 to review ongoing research activities and strategic priorities across the...